Join us at Fraunhofer Institute for Cell Therapy and Immunology IZI on 28–29 August where the spotlight will be on adeno-associated virus (AAV) and protein production and equipment: ÄKTA™ systems plus imaging and Biacore™ systems.
Engage in scientific presentations, hands-on sessions with Cytiva equipment, and take the opportunity to take a tour through the institute.
Where we’ll host you:
Fraunhofer-Institut für Zelltherapie und Immunologie IZI
Perlickstr. 1, 04103 Leipzig, Germany
In collaboration with:

Register to attend
Insert text
Panel 3 header
Insert text

Speakers and topics
The gallery of speakers invited to host talks and speak to you on all things SPR, along with the subject area they will be coming to speak about. This list will be updated with each new wave of speaker announcements.
- Phil Addis, Sygnature Discovery - Pushing the limits: Establishing scalable SPR assays for molecular glue discovery
- Jason Baardsnes, National Research Council Canada - SARS Cov2 – Ace2 binding assays for kinetics, selection and QC
- Aurelie Castan, Novartis Pharma AG - Characterization of antibody binding to FcɣRIIIa by SPR and affinity chromatography MS
- Göran Dahl, AstraZeneca
- Gregory Descrescenzo, Montreal Poltyech
- Tim van den Hooven, Genmab - FcRn characterization: an odyssey to (and through) qualification
- Ute Jucknischke, Roche Diagnostics - Kinetics with native biomarkers – The reality check for antibodies
- David Kallenberg, UCB - Fragment screening on the Biacore 8K+ and an evaluation of the Biacore Intelligent Analysis™ machine learning software
- Matthias Kania, Boeringer Ingelheim Pharma GmbH & Co. K - Applications of Biacore technology in a regulated biopharmaceutical environment
- Matthias Knape, Boeringer Ingelheim Pharma GmbH & Co. K - Applications of Biacore technology in a regulated biopharmaceutical environment
- Patrik Nygren, BioArctic
- Dr. Hanno Sjuts, Sanofi
- Pedro M.F. Sousa, iBet - Extract2chip - Bypassing protein purification in drug discovery using SPR
- Rob Staehlin, Purdue University - Lipid-protein interactions in virus assembly, budding and virus spread
- David Zollman, University of Dundee - Expanding the biophysical toolbox for targeted protein degradation drug discovery
Panel 4 with video
Insert text
Insert text
Meet our speakers

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker name
Job & company
Speaker details

Mojtaba Parvisi
Job & company
Speaker details
Speaker Name
Job & company
Speaker details
Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details

Speaker Name
Job & company
Speaker details
Speaker Name
Job & company
Speaker details
Agenda
Subject to minor changes leading up to the event date.
| Time | Event | Speaker |
|---|---|---|
| 09:00 - 09:15 | Welcome | Prof. Dr. Ulrike Köhl (Fraunhofer IZI) |
| 09:15 - 09:30 | Introduction | Marialuisa Casamenti (Cytiva) |
| 09:30 - 10:15 | AAV purification – tools and digital solutions for an efficient and robust separation | Lutz Mathe (Cytiva) and Franco Klingberg (Cytiva) |
| 10:15 - 10:45 | Design of experiments is important for scientific improvements | Dr. Peter Paul Heym (Sum Of Squares – Statistical Consulting) |
| 10:45 - 11:00 | Q&A | |
| 11:00 - 11:30 | Tips for straightforward protein chromatography | Stephanie Runde (Cytiva) |
| 11:30 - 11:45 | Optimization of purification processes using Amersham™ ImageQuant™ 800 and IQTL analysis software | Linda Keller (Cytiva) |
| 11:45 - 12:45 | Hands-on Q&A featuring Biacore™, ÄKTA™, imaging systems, and institute tour | |
| 12:45 - 13:45 | Break | |
| 13:45 - 14:45 | More hands-on Q&A and institute tour | |
| 14:45 - 15:15 | From transient to stable: How cell lines influence AAV manufacturing and product quality | Mischa Schwendy (Cytiva) |
| 15:15 - 15:45 | Generation of monoclonal antibodies: accompanied by SPR and ELISA | Dr. Martin Kleinschmidt (Fraunhofer IZI) |
| 15:45 | Q&A |
| Time | Event | Speaker |
|---|---|---|
| 9:00 - 11:00 | In-depth, hands-on session for protein purification | |
| 11:00 - 13:00 | Individual conversations. Interested? Book yours in advance via the registration form |
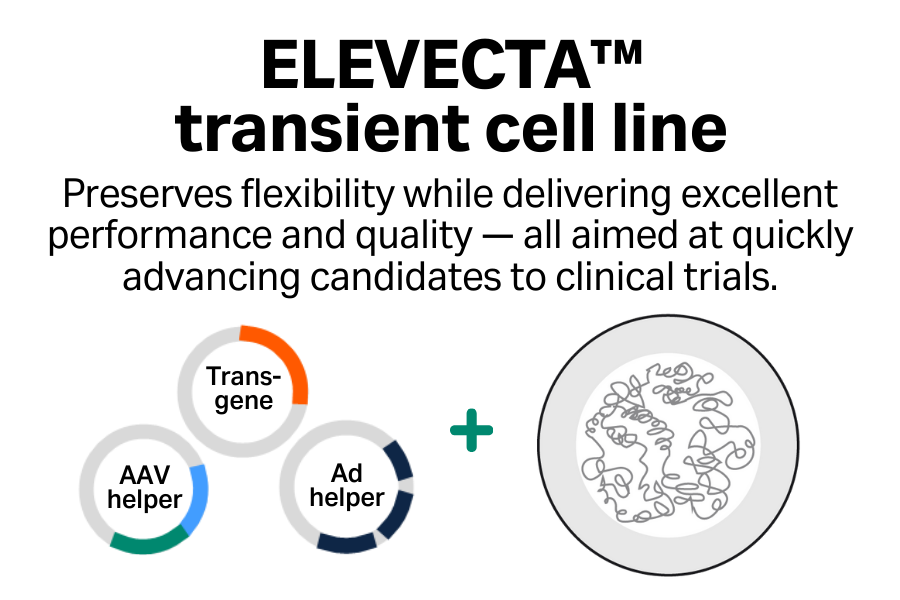
Excellent performance
Achieve high titers of infectious virus, which you can boost further by using enhancers.
Enhanced product quality
Minimize DNase-resistant host cell DNA inside capsids.
Innovative
Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
Collaborative
Benefit from a comprehensive suite of regulatory support services.

Excellent performance
Achieve high titers of infectious virus, which you can boost further by using enhancers.
Enhanced product quality
Minimize DNase-resistant host cell DNA inside capsids.
Innovative
Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
Collaborative
Benefit from a comprehensive suite of regulatory support services.
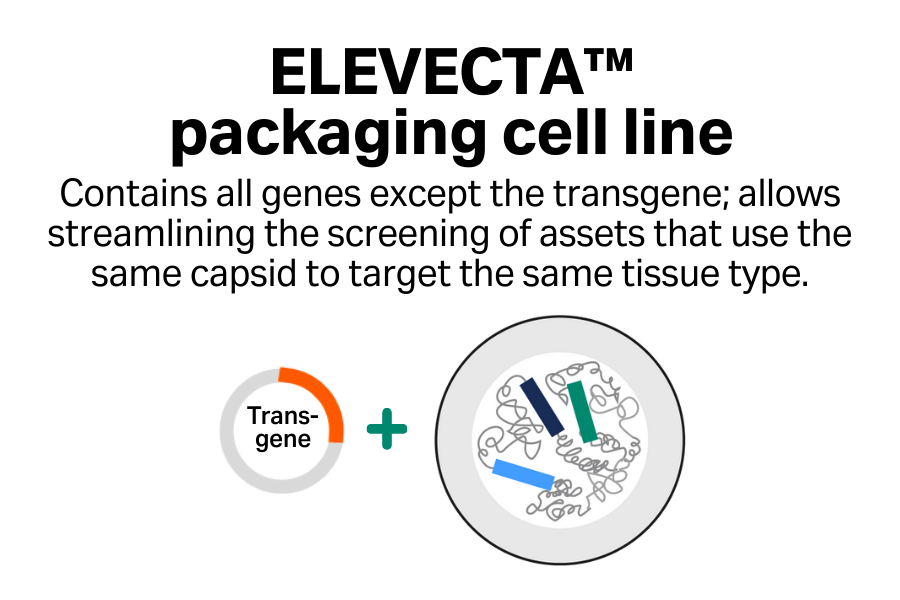
Streamlined screening
Efficiently screen multiple assets that target the same tissue type.
Cost-effective approach
Reduce your plasmid costs with single-plasmid transfection compared with triple transfection.
One cell line to clinic
Go to clinic with one cell line and simplify your regulatory asks when you’re targeting the same tissue type across your programs.
