High-throughput sterilizing-grade filters for challenging feeds
See how Supor™ Prime 0.2 micron filters perform with high-concentration mAbs and other drugs.
When it comes to high-concentration mAbs, proteins, or other biotech drugs, every drop is precious — so preventing product loss is crucial at every filtration step. These steps can also pose unique challenges. High particulate loads during ultrafiltration and diafiltration require larger filter area to prevent blocking, while the high viscosity of your product can slow the final sterile filtration steps to a crawl.
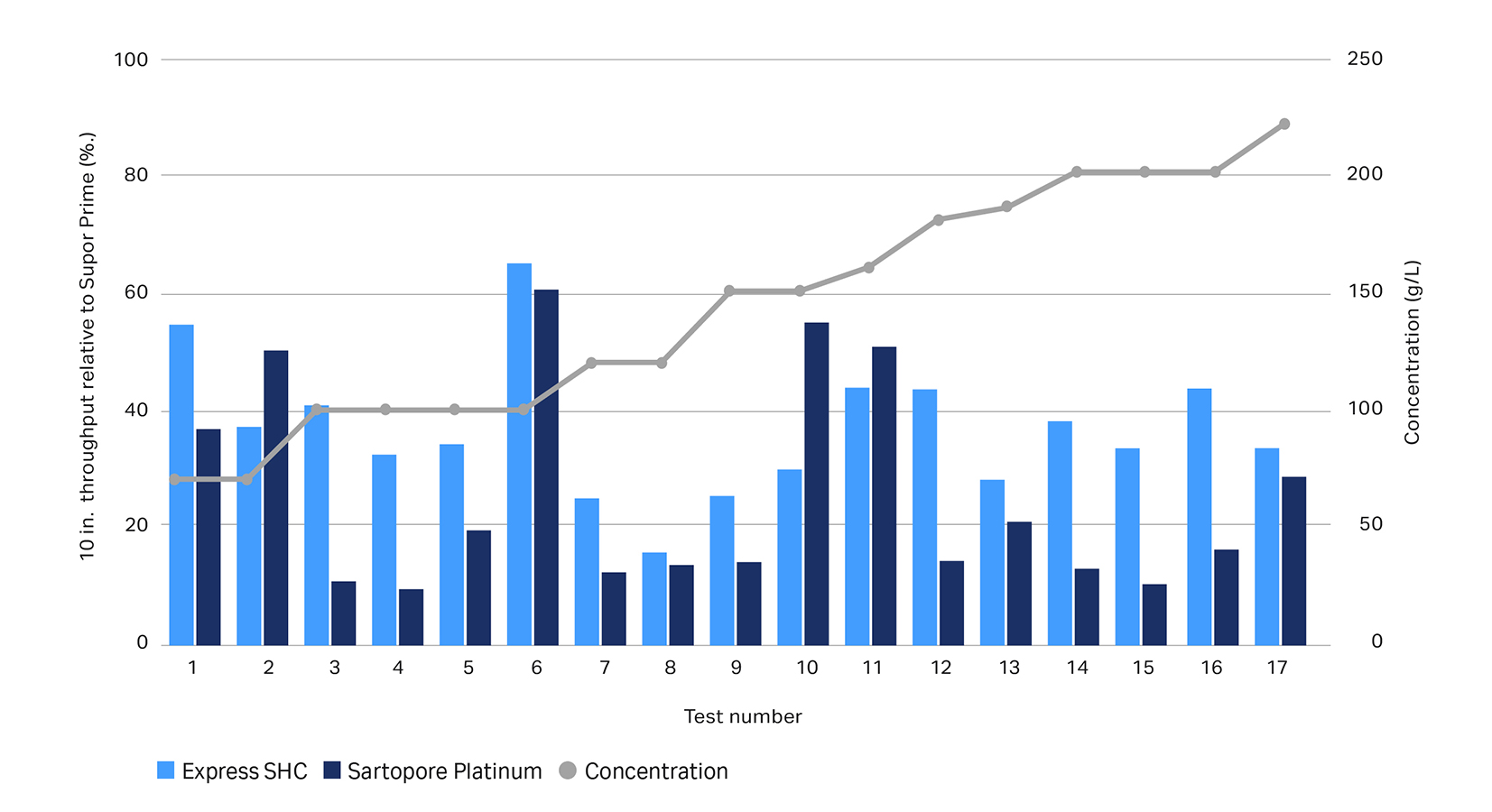
We designed Supor Prime 0.2-micron filters specifically to address these challenges. These PES filters deliver, on average, twice the volumetric throughput of comparable products (Fig. 1) — improving yields and process efficiency. And they’re scalable, so you can grow your process from clinical development up to manufacturing.
 |
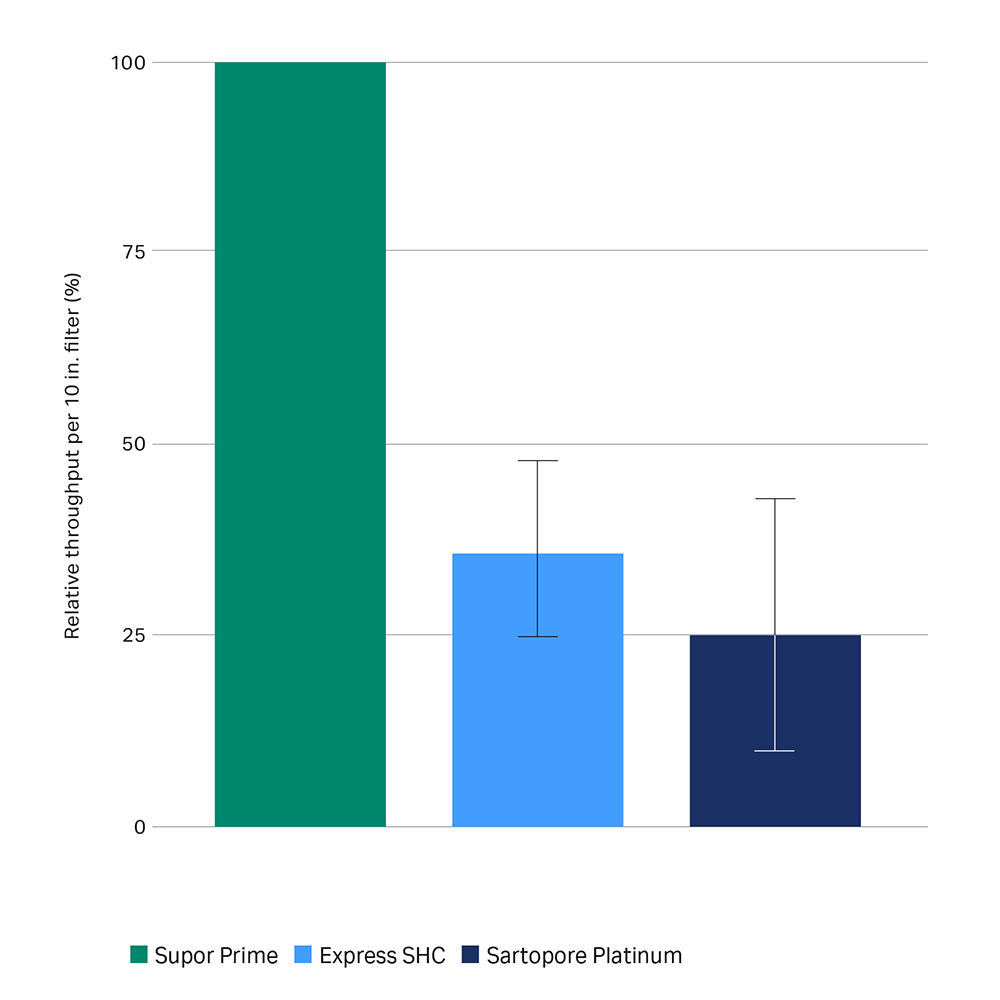 |
Figure 1. Relative throughput performance of Supor Prime, Express® SHC, and Sartorpore® Platinum filters. Left, relative throughput per 254 mm (10 in.) filter. Each data point is n =1, tested in parallel with other filters and scaled up using published filtration areas. Individual feed comparisons may vary upon replication. Right, average relative throughput performance for per 254 mm (10 in.) filter. Each data point scaled up using published filtration areas. Error bars, ± s.d.; p < 0.01, two-tailed paired Student’s t-test; n = 17.
You can use Supor Prime filters for:
- Filtration after diafiltration or ultrafiltration
- Filtration of bulk drug substance
- Final formulation/fill finish filtration
Fill out the form to read the application note and request a sample.
*required fields
Form title
Form sub title
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .



