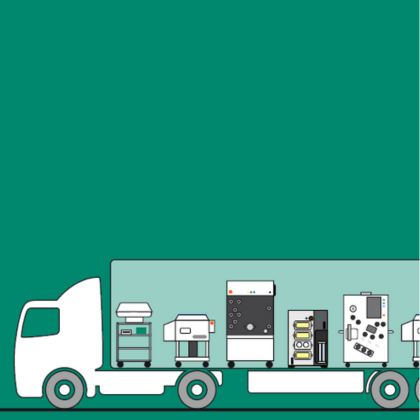
KUBio™ box trailer:
Rapidly deployable capacity
When and where you need it to get therapies to patients faster.
Would you like to learn more about Cytiva’s rapidly deployable mRNA manufacturing capacity?
To receive your personal voucher, please read our Terms and Conditions and simply fill out the contact form below.
Explore how the KUBio™ box trailer is revolutionizing advanced therapy manufacturing. This modular, prefabricated biomanufacturing facility is engineered for rapid deployment and scalability, making it ideal for cell and gene therapy, mRNA production, and more.
Powered by the FlexFactory™ manufacturing platform, KUBio box trailer integrates process equipment, automation, and digital solutions into a compact, GMP-compliant cleanroom environment—helping companies accelerate time-to-market and simplify tech transfer while saving on facility space.
Key Features:
- Modular cleanroom infrastructure for advanced therapies
- Seamless integration with FlexFactory™ bioprocessing equipment
- Designed for speed, flexibility, and regulatory compliance
- Ideal for cell therapy, gene therapy, and mRNA production
- Scalable from clinical to commercial manufacturing

Class is in session
Next generation mRNA is on the rise.
Hear our own Dave Sokolowski talk about mRNA manufacturing basics.

Watch CPI discuss their advances in mRNA via the Cytiva FlexFactory Platform
Modular manufacturing for scalable RNA-LNP therapies.
Supported by the UK Vaccine Taskforce, the Centre for Process Innovation (CPI) has established the UK’s first GMP-ready manufacturing site to support a full mRNA-LNP development and manufacturing process. .
mRNA production using the Wave25 rocker
The intensification of interest in mRNA has spurred the demand for mRNA manufacturing capabilities. We will show that the ReadyToProcess WAVE™ 25 Rocker system offers a complete platform for the GMP linearization of template DNA and subsequent production of mRNA..
mRNA lipid nanoparticle encapsulation and purification
Our work showed:
- An enhanced green fluorescent protein (eGFP2) mRNA encapsulation process that successfully scaled up to a production batch size of 49 L (1.9 g).
- A downstream purification process that successfully scaled to an output batch size of 15 L (1.6 g) of final product.
- A formulation step with a 94% mRNA recovery.
- A A TFF purification step with a yield > 100%.
Bionova Scientific expands capacity and enters advanced therapies market
Bionova Scientific, a contract development and manufacturing organization (CDMO), is entering the advanced therapy manufacturing space. The company is installing its third FlexFactory manufacturing platform from Cytiva, a Danaher company and a leader in the life sciences industry, to help meet the surging demand for advanced therapies, while maintaining their core monoclonal antibody (mAb) business.
Rapidly deployable mRNA FlexFactory™ facility
In this video, you’ll get a behind-the-scenes look at how KUBio box trailer supports the evolving needs of advanced therapy manufacturers. From rapid deployment and modular design to integrated bioprocessing and digital control, KUBio box trailer is engineered to help you launch faster, operate smarter, and stay compliant.