Choose a PUPSIT solution that suits the way you work
Manual:
- Standardized installation
- Easy to use
- Configurable design
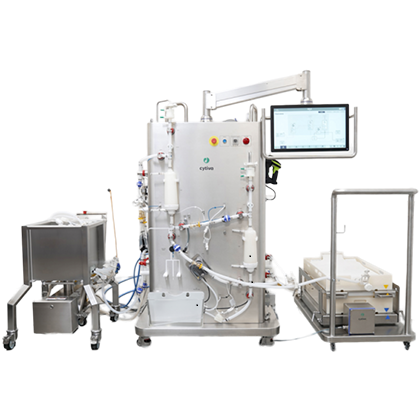
Automated:
- Consistent processing
- Enhanced product recovery
- Integrates into your existing control system
Standard filter sets:
- Shorter lead times
- Simplified development and testing
- Optimized designs
Find out more today!
Pre-use post-sterilization integrity testing
PUPSIT
Dedicated products, implementation services and ongoing support to help you achieve your goals.
What is PUPSIT, and why does it matter?
PUPSIT helps ensure the sterility of aseptically-filled medicinal drug products. It determines the integrity of the sterilizing-grade filter and assembly, including filter housing, support structure, and connections, after the assembly has been installed and sterilized but before it is used to filter product.
PUPSIT guards against filter damage during shipping, handling, installation, and sterilization prior to use. A damaged filter could result in a non-sterile product, which can ultimately affect drug safety.

Meet our range of PUPSIT solutions
Implementing PUPSIT effectively requires a delicate balance.
Work with Cytiva’s process development team to get it right from the start. Our manual, automated and custom solutions and ongoing support help give you peace of mind as you implement PUPSIT.
Get your tailored solution…











