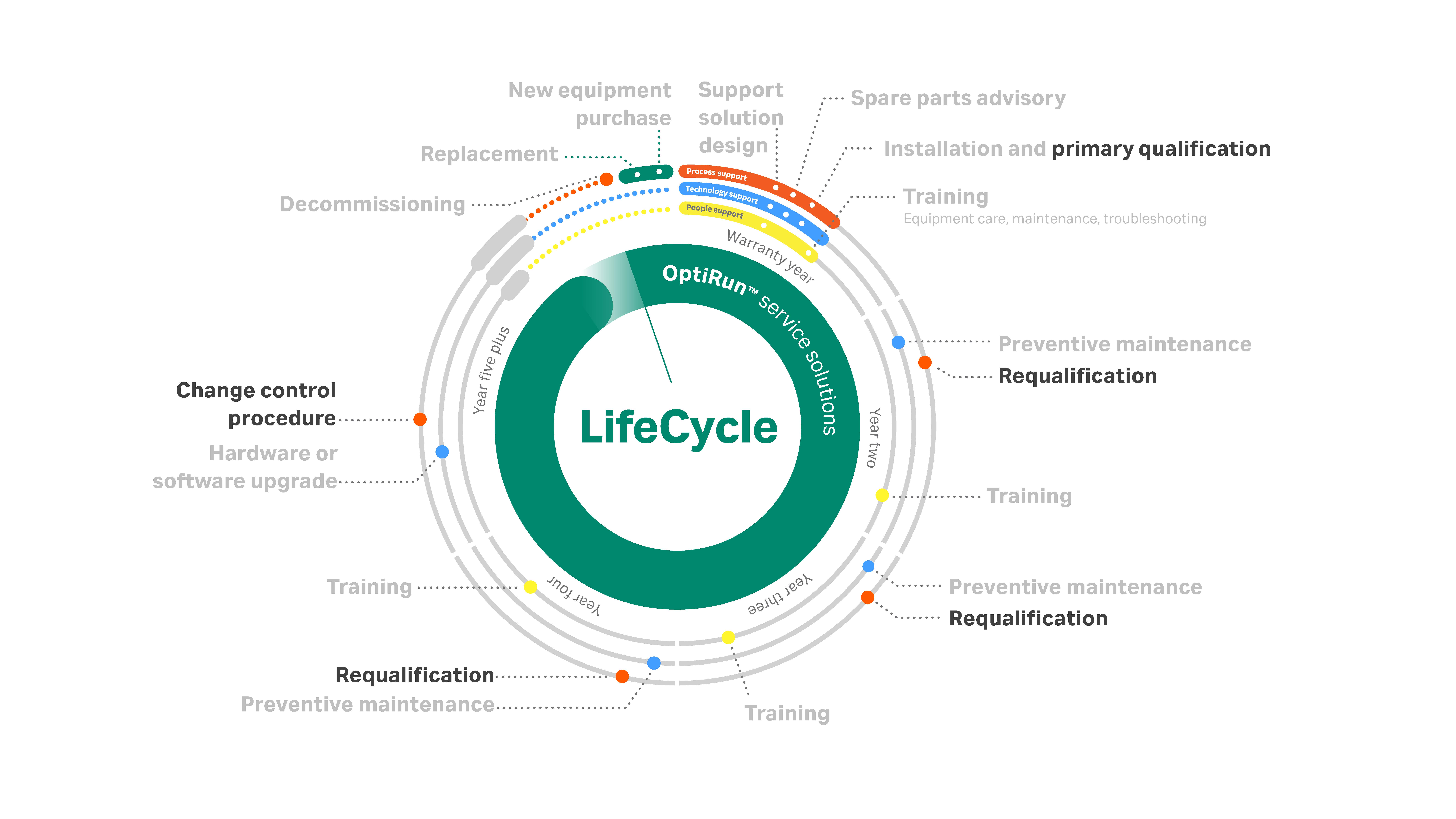
 Our instrument qualification services follow the complete equipment LifeCycle. Embark on your GMP manufacturing journey with our qualification knowledge by your side.
Our instrument qualification services follow the complete equipment LifeCycle. Embark on your GMP manufacturing journey with our qualification knowledge by your side.
- Maintain compliant systems - Rely on our dependable IQ/OQ protocols for compliance with regulatory standards.
- Keep your equipment qualified - When your equipment is repaired, relocated, upgraded, or modified, Cytiva can help you keep it in a qualified state with requalification and change control procedure services.
- Contribute to sustainability - Reduce your risk, save time and CO2 emissions with digital delivery of qualification protocols.
- Enjoy consistent support - Secure your process with a qualification contract.
Leverage the experience of the organization that designed your equipment.
To learn about our complete LifeCycle solutions, click here.
Fill out this form to learn more
*required fields
Insert text
Acrodisc® Syringe Filters
Reliable Results with Superior Protection
Insert text
Form title
Form sub title
Insert text
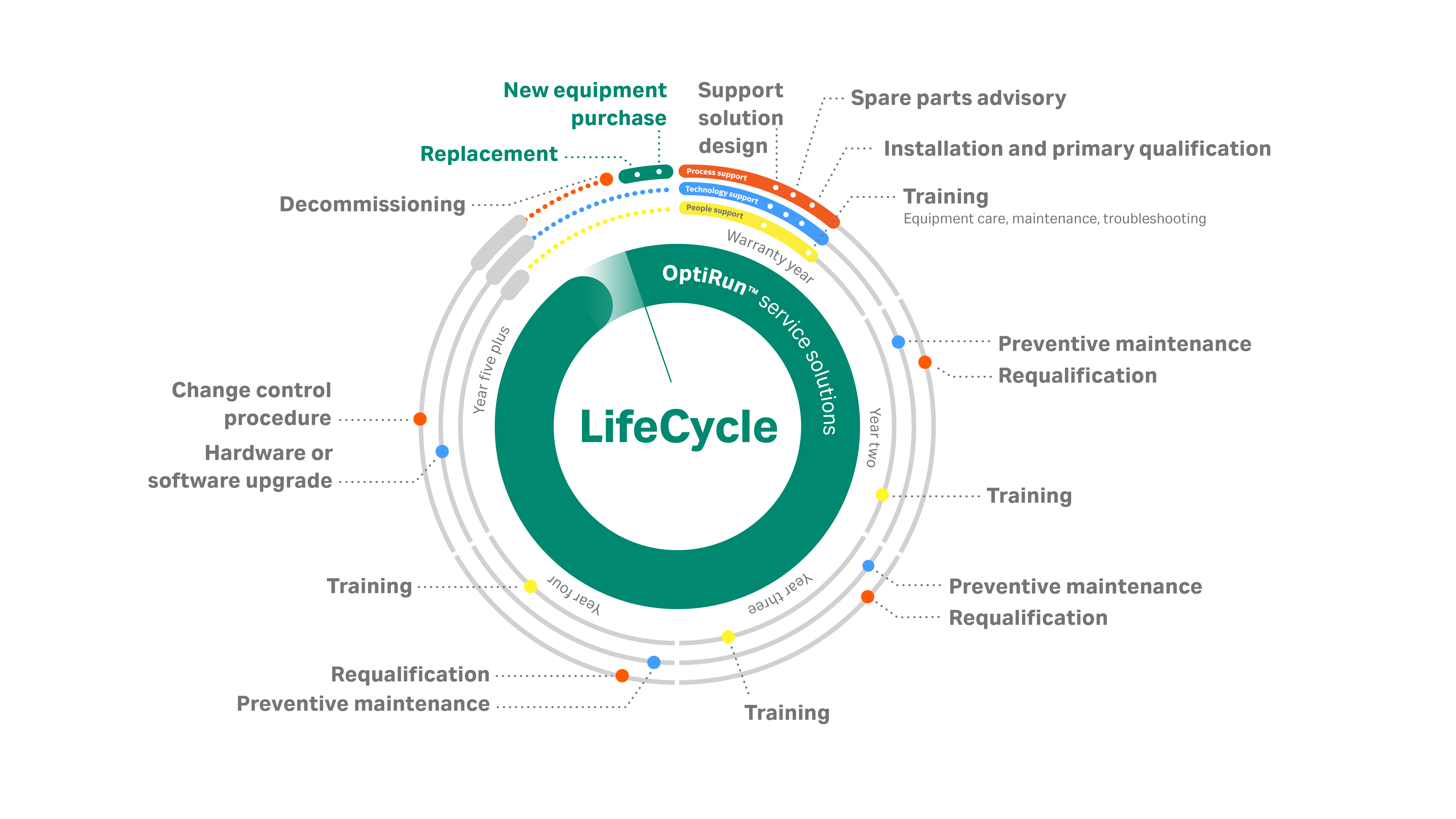
The OptiRun™ service solutions portfolio was designed to provide continuous support for your equipment through every critical stage. From the moment of purchase and through the life of your equipment, our proactive LifeCycle support, which is presented as a single word to denote continuous care, ensures your outcomes are met.
Navigate our LifeCycle offerings by selecting the hyperlinks below.
Bookmark this page to learn about each design component as we reveal them separately.
- Service solution design
- Spare parts advisory
- Qualification services
- Preventive maintenance
- Equipment care and troubleshooting training
- Equipment connectivity and monitoring
Panel 3 header
Insert text

Follow our connected mAb perfusion process
Insert text
Insert text
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .


