Panel 1 header
Insert text
Form title
Form sub title
Insert text
Panel 3 header
Insert text

Panel 3a header
Insert text
Panel 4 with video
Insert text
Insert text
Meet our speakers

Emmanuel Abate,
President Genomic Medicine,
Cytiva

Stephanie Bourin,
Senior Product Manager,
Cytiva

Samuel Clarke,
Director R&D,
Cytiva

Marine De Lageneste,
Biology Team Leader,
Cytiva

Aaron Dulgar-Tulloch,
Chief Technology Officer, Genomic Medicine,
Cytiva

Bertrand Foucaut,
Senior Product Manager,
Cytiva

Federico Franchi,
R&D Manager MBO Biology,
Cytiva

Angela Johnson,
Global Regulatory and Compliance Leader,
Cytiva

Sadik Kassim,
Chief Technology Officer,
Danaher

Brent Lieffers
Senior Director of Innovation Advocacy,
Cytiva

Stella Park,
Associate Scientist, Product Development,
Cytiva

Mojtaba Parvizi,
Global Fast Trak Leader — Cell Therapy,
Cytiva

Scott Ripley,
Business Leader, Nucleic Acid Therapeutics and Precision Nanosystems,
Cytiva

Mitchel Sivilotti,
President and Chief Executive Officer,
OmniaBio
Meenakshi Swaminathan,
Associate Scientist, Product Development,
Cytiva

Jim Thompson,
Director of R&D,
West Pharmaceutical Services

Martin Westberg,
Vice President, Cell Therapy,
Cytiva

George White,
Cell Therapy Product Management Leader,
Cytiva
Robert Young,
Senior Engineer, Systems,
Cytiva
Agenda
Wednesday 29 May, 2024
| Time | Event | Venue | Title |
|---|---|---|---|
| 7:00-8:30 PM | Welcome Poster | ??? | End-to-end manufacturing of autologous CAR T cell therapies with a new cell therapy manufacturing platform. |
| 7:00-8:30 PM | Poster | ??? | Regulatory T cell expansion in the Xuri™ cell expansion system W25 |
| 7:00-8:30 PM | Poster | ??? | Optimization of multiplex CRISPR-Cas9 editing of human primary T cells using lipid nanoparticles (LNPs) and subsequent off-target evaluation |
| 7:00-8:30 PM | Poster | ??? | Strategies for producing clinical and commercial RNA-LNP drug products |
Thursday 30 May, 2024
| Time | Event | Venue | Title |
|---|---|---|---|
| 1:15-1:30 PM | Welcome Global showcase | ??? | New containment solutions for automated aseptic filling for gene therapy |
| 1:30-1:45 PM | Global showcase | ??? | An introduction of the new, end-to-end solution from Cytiva to manufacture autologous CAR T therapies at commercial scale |
| 6:00-7:00 PM; | Corporate session | ??? | Deliver what's next: fueling the future of autologous CAR T therapy |
| 6:00-7:30 PM | Poster | ??? | Closed, semi-automated harvest of iPSC scale-up workflow using the Sepax™ C-Pro with CultureWash protocol |
| 6:00-7:30 PM | Oral abstract presentation | ??? | An introduction of the new, end-to-end solution from Cytiva to manufacture autoEfficient gene editing in CD34+ hematopoietic stem and progenitor cells using non-viral lipid nanoparticles |
Friday 31 May, 2024
| Time | Event | Venue | Title |
|---|---|---|---|
| 12:30-12:45 PM | Product showcase | ??? | Ex Vivo Engineering of Primary Cells Using RNA-Lipid Nanoparticles and Scale-Up Manufacturing for Cell and Gene Therapies |
| 3:45-4:45 PM | Launch and patient access concurrent session | ??? | Industrializing manufacturing and distribution |
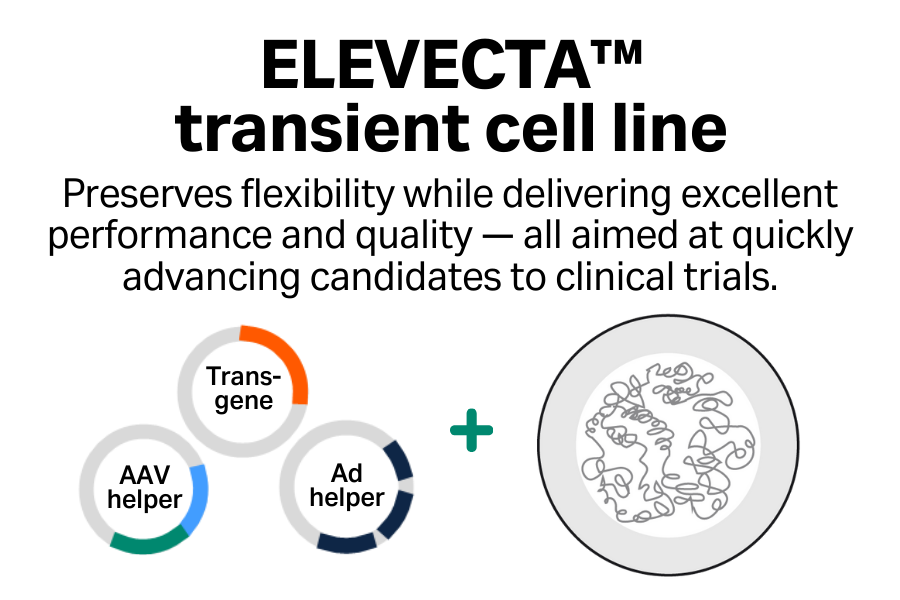
Excellent performance
Achieve high titers of infectious virus, which you can boost further by using enhancers.
Enhanced product quality
Minimize DNase-resistant host cell DNA inside capsids.
Innovative
Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
Collaborative
Benefit from a comprehensive suite of regulatory support services.

Excellent performance
Achieve high titers of infectious virus, which you can boost further by using enhancers.
Enhanced product quality
Minimize DNase-resistant host cell DNA inside capsids.
Innovative
Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
Collaborative
Benefit from a comprehensive suite of regulatory support services.
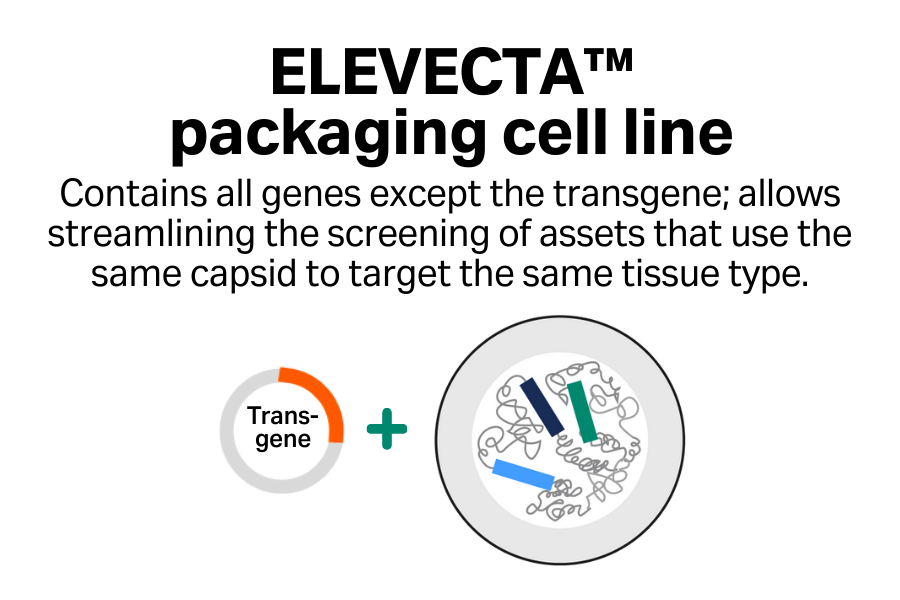
Streamlined screening
Efficiently screen multiple assets that target the same tissue type.
Cost-effective approach
Reduce your plasmid costs with single-plasmid transfection compared with triple transfection.
One cell line to clinic
Go to clinic with one cell line and simplify your regulatory asks when you’re targeting the same tissue type across your programs.