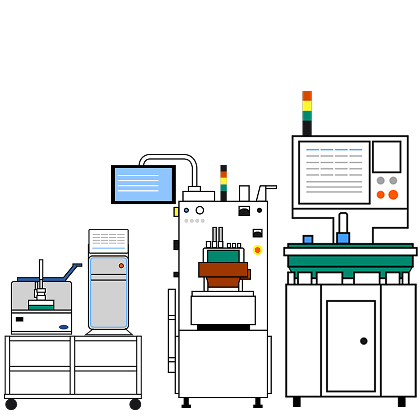
Unlock the full potential of your adherent cell culture processes with the iCELLis™ bioreactor platform.
iCELLis™ bioreactors offer unmatched scalability, automation, and process consistency – empowering you to move from R&D to GMP manufacturing faster and more efficiently than ever before.
Why iCELLis™ bioreactors?
- Proven performance: used in 6 approved therapies, including Zolgensma for treatment of spinal muscular atrophy
- Scalable innovation: seamless scale-up from 0.5 to 500 m2 in a single platform
- GMP-readiness: closed single-use system, 21 CFR Part 11 compliant software that has undergone 100+ hours of user testing to optimize ease-of-use
- Versatility: 90+ publications demonstrating successful production of AAV, Lentivirus, oncolytic virus, exosomes, human vaccines, and more
Your goals, supported worldwide
From proof-of-concept to commercial launch, our team of Field Application Specialists and Fast Trak™ process development services partner with you every step of the way - bringing global expertise with local, hands‑on support.