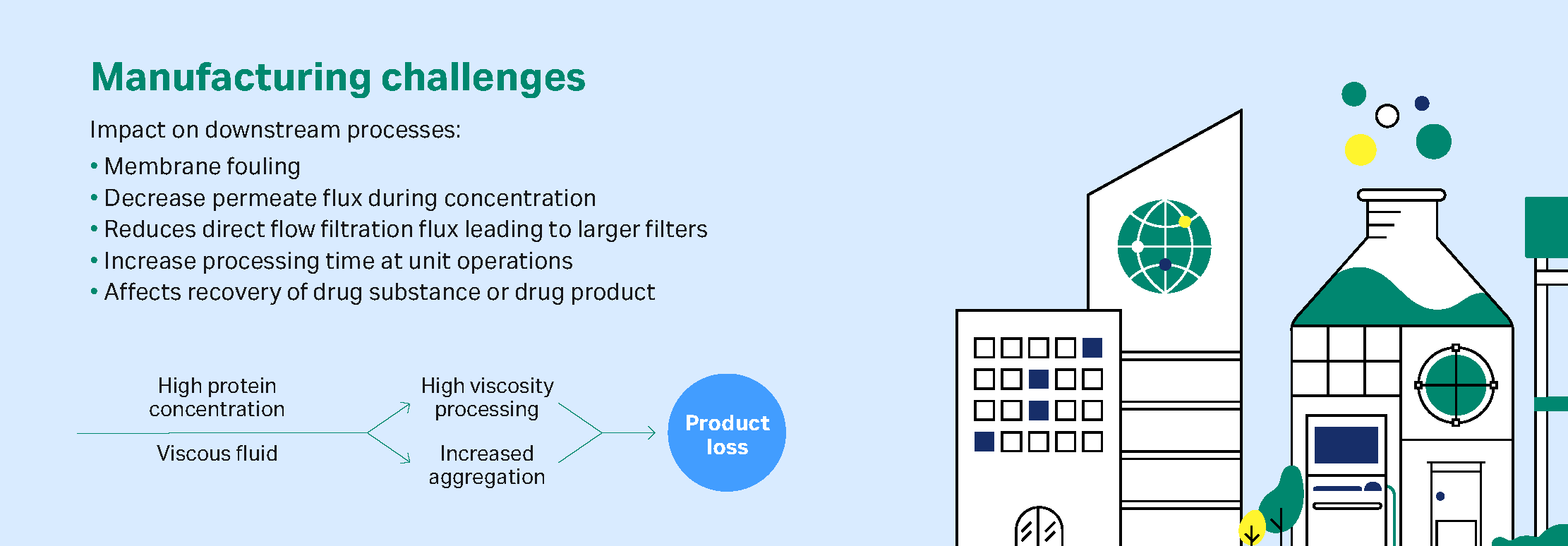
Manufacturing challenges with high concentration biologics
Subcutaneous delivery of biologics such as monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs) and recombinant proteins is becoming commonplace in new treatments for autoimmune diseases, cancers, and diabetes.
Download the white paper, 'Manufacturing challenges with high concentration biologics', today.
Understand the challenges. Be ahead of the game. Discover what you could change.
Fill out this form to download the whitepaper
*required fields
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer posuere erat a ante.
Acrodisc® Syringe Filters
Reliable Results with Superior Protection
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Form title
Form sub title
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer posuere erat a ante.
Klaus Hochleitner, PhD
Global Technology Lead, Diagnostics, Cytiva
Dr. Hochleitner is a cell and molecular biologist and has covered different roles in the field of membrane research and development and developing diagnostic tests on solid surfaces. He has worked in the field of lateral flow tests for more than 25 years.

Klaus Hochleitner, PhD
Global Technology Lead, Diagnostics, Cytiva
Dr. Hochleitner is a cell and molecular biologist and has covered different roles in the field of membrane research and development and developing diagnostic tests on solid surfaces. He has worked in the field of lateral flow tests for more than 25 years.
Introduction
Global Technology Lead, Diagnostics, Cytiva Dr. Hochleitner is a cell and molecular biologist and has covered different roles in the field of membrane research and development and developing diagnostic tests on solid surfaces. He has worked in the field of lateral flow tests for more than 25 years.
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .


