About the event
There’s no substitute for an in-person event.
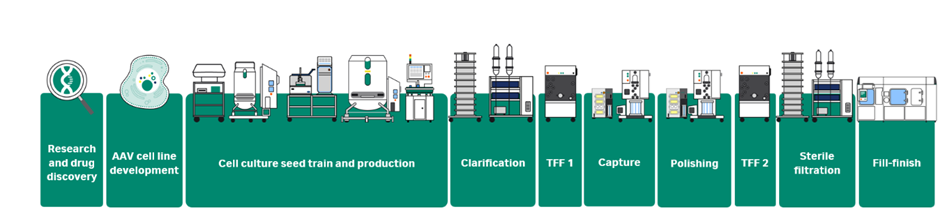
Join us on Tuesday 19 November from 12:30 to 18:00 CET, to hear about the latest innovations in upstream viral vector manufacturing, from cell lines to bioreactors, and how industry experts successfully use them to intensify their process.
If you’re looking to significantly reduce your time to milestone or market by speeding up process development and scale-up, you won’t want to miss hearing about our Fast Trak™ services.
We will cap off the day with some refreshments while you try to master our “Viral Blast” video game.
Where we’ll host you:
Biotech Training Facility
Sylviusweg 70
2333 BE Leiden
Netherlands
Don't miss out
Spots are limited. Save yours by registering today
Book your place today
Insert text
Acrodisc® Syringe Filters
Reliable Results with Superior Protection
Insert text
Form title
Form sub title
Insert text
Event Agenda
| 12:30 | Registration and welcome lunch |
| 14:00 | Opening: Advance gene therapies at the speed of light |
| 14:15 | Fueling viral vector production with ELEVECTA™ tailored cell lines |
| 14:45 | Cytiva bioreactors and support for adherence and suspension cell growth |
| 15:20 | Innovative Efficiency: Fixed Bed Bioreactor Technology Combined with Viral Sensitizers for Increased Production of AAV (Oliver Varette, Virica Biotech) |
| 15:40 | Coffee break |
| 16:00 | Media Optimisation: How to choose the right media and feed strategy for your viral vector |
| 16:30 | Fast-Trak services and digital solutions: Your helping hand in advanced therapeutics: Solutions support from process development to manufacturing |
| 17:00 | Evening refreshments and game contest: be the best at viral blast video game |
About Cytiva
Cytiva, now with the life sciences business from Pall Corporation, is a global life sciences leader dedicated to helping customers discover and commercialize the next generation of therapeutics.
Together, we bring dedicated technical expertise and a broad portfolio of tools and technologies that enable the development, manufacture, and delivery of transformative medicines to patients. Visit cytiva.com to learn more.
Panel 3 header
Insert text

Follow our connected mAb perfusion process
Insert text
Insert text
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .


