Panel 1 header
Insert text
Form title
Form sub title
Insert text
Acrodisc® Syringe Filters
Reliable Results with Superior Protection
Insert text
Form title
Form sub title
Insert text
ELEVECTA™ cell line portfolio for AAV manufacturing
Beyond supporting your gene therapy processes with our technology and application knowledge, we're passionate about advancing solutions in areas where the status quo isn’t good enough. That's why we're excited to tell you about our ELEVECTA™ cell lines ― a significant advancement in viral vector manufacturing.

The ELEVECTA™ cell line portfolio combines innovative design and deep expertise to deliver transient, packaging, and producer options that provide competitive performance, high quality, and flexibility.
- Fit for purpose: Choose the option that makes sense for your stage, asset mix, and organizational goals.
- Flexible: Enjoy competitive terms and the freedom to transition between cell lines as needs evolve.
- Enhanced product quality*: Minimize host cell DNA inside capsids, which is resistant to nucleases.
- Innovative: Access advances in cell line engineering informed by deep industry know-how.
- Collaborative: We’re more than a supplier. Benefit from a comprehensive suite of regulatory support services designed to expedite your success in advancing to and through clinical trials.
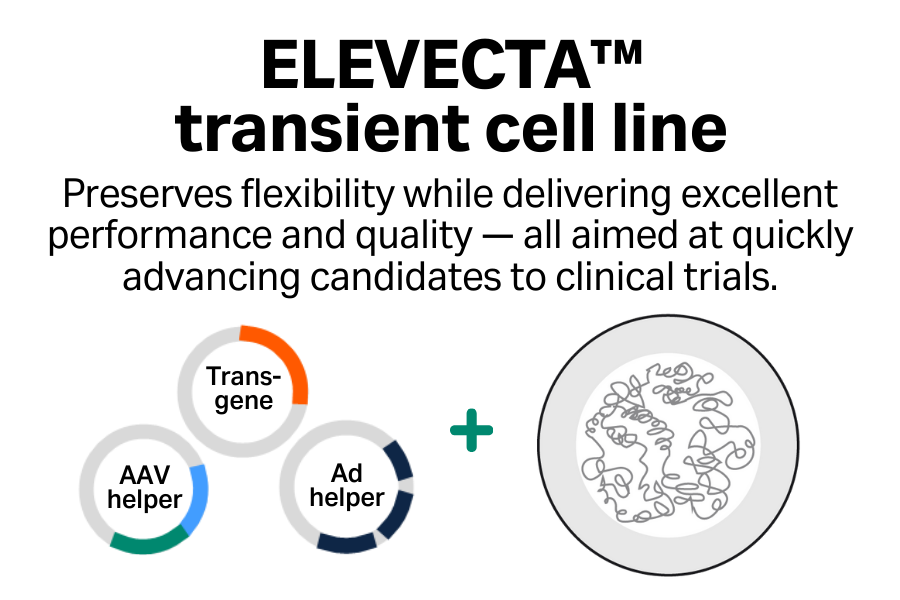
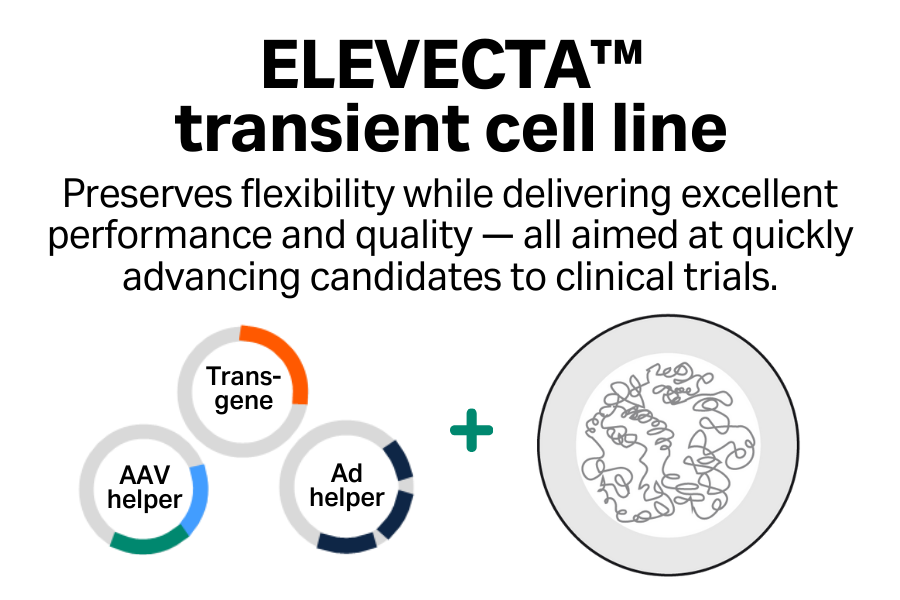
- Excellent performance: Achieve high titers of infectious virus, which you can boost further by using enhancers.
- Enhanced product quality*: Minimize DNase-resistant host cell DNA inside capsids.
- Innovative: Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
- Collaborative: Benefit from a comprehensive suite of regulatory support services.
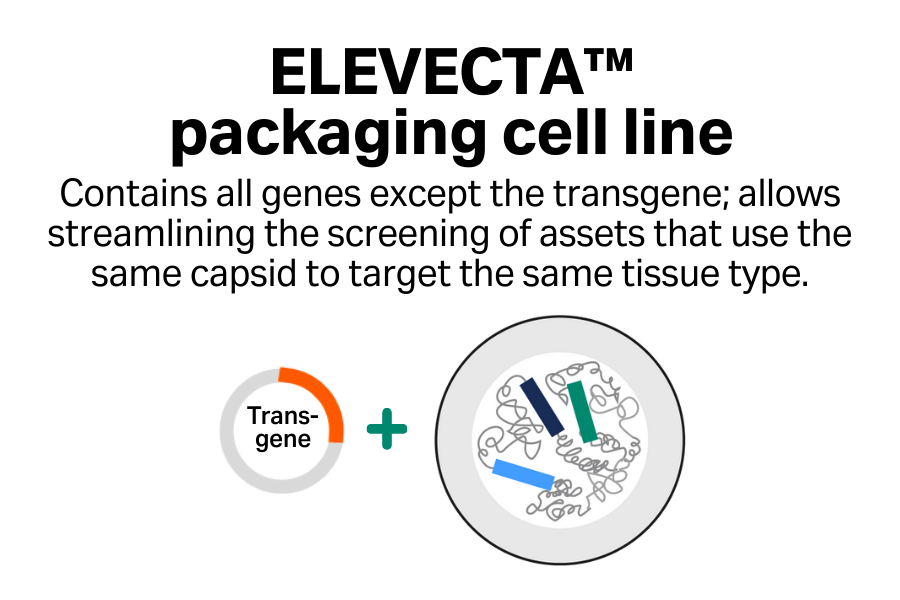
- Streamlined screening: Efficiently screen multiple assets that target the same tissue type.
- Cost-effective approach: Reduce your plasmid costs with single-plasmid transfection compared with triple transfection.
- One cell line to clinic: Go to clinic with one cell line and simplify your regulatory asks when you’re targeting the same tissue type across your programs.
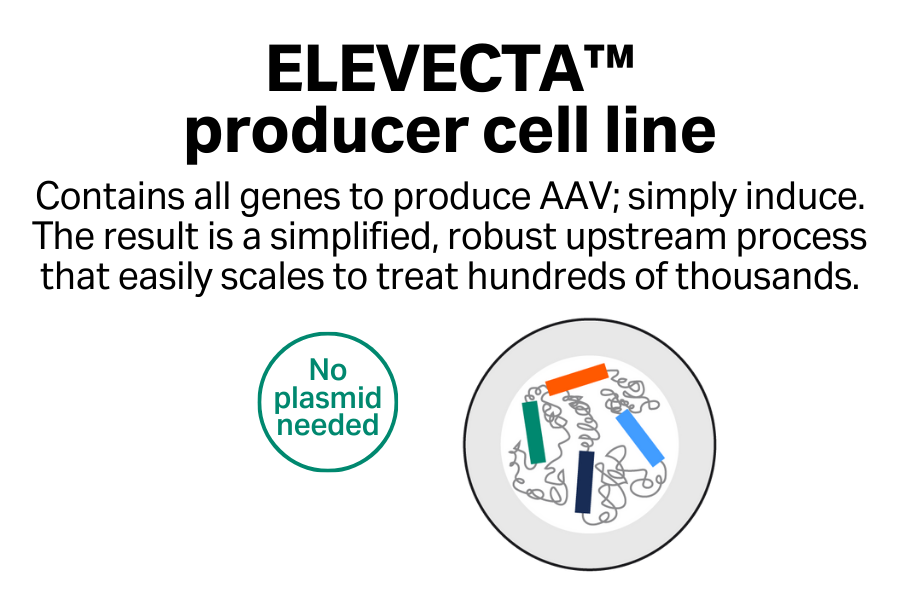
- Simplified manufacturing process: Replace multi-step transfection with one-step induction.
- Easy scalability: Scale up seamlessly to achieve large quantities of AAV material.
- Enhanced reproducibility: Produce AAV from a monoclonal cell line, enabling batch-to-batch consistency.
- Reduced COGS: Skip the need for transfection reagent and any plasmid DNA, which are the most expensive raw materials.
*Minimizing host cell DNA is a feature for all cell lines. ELEVECTA transient cell line and hcDNA feature available soon.
Panel 3 header
Insert text

Follow our connected mAb perfusion process
Insert text
Insert text
- Excellent performance: Achieve high titers of infectious virus, which you can boost further by using enhancers.
- Enhanced product quality: Minimize DNase-resistant host cell DNA inside capsids.
- Innovative: Access advances in cell line engineering across the entire portfolio to support transition between cell lines.
- Collaborative: Benefit from a comprehensive suite of regulatory support services.
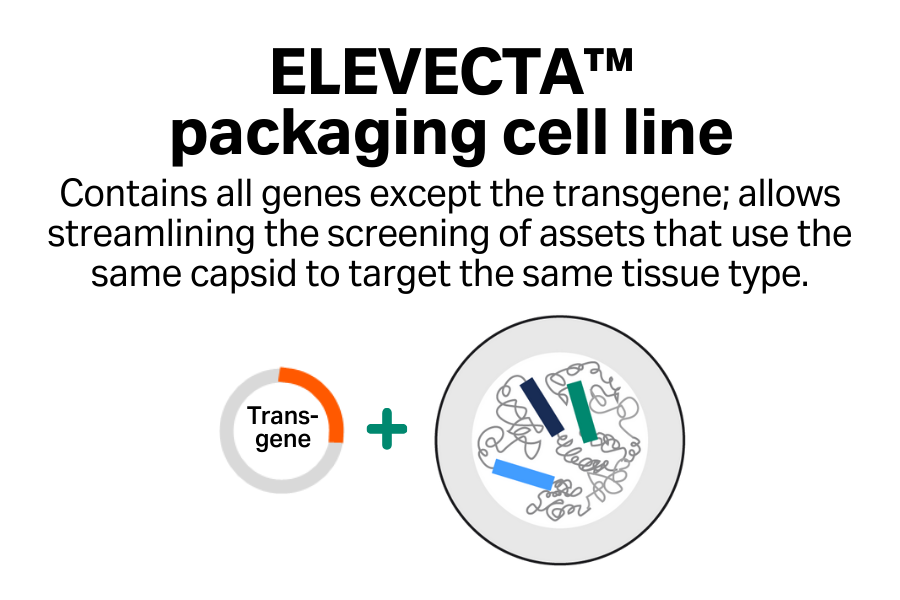
- Streamlined screening: Efficiently screen multiple assets that target the same tissue type.
- Cost-effective approach: Reduce your plasmid costs with single-plasmid transfection compared with triple transfection.
- One cell line to clinic: Go to clinic with one cell line and simplify your regulatory asks when you’re targeting the same tissue type across your programs.

- Simplified manufacturing process: Replace multi-step transfection with one-step induction.
- Easy scalability: Scale up seamlessly to achieve large quantities of AAV material.
- Enhanced reproducibility: Produce AAV from a monoclonal cell line, enabling batch-to-batch consistency.
- Reduced COGS: Skip the need for transfection reagent and any plasmid DNA, which are the most expensive raw materials.
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .
Curious about continuous biomanufacturing? Join the Cytiva project team vlog series as we document our proof of concept trial and provide insights to help you efficiently plan and execute your project .


