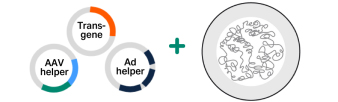
Praesent lacus arcu, mollis ut volutpat pharetra, vulputate quis massa. Nam interdum fringilla bibendum. Maecenas non odio dui. Suspendisse eros ex, ullamcorper et dui non.
Sed feugiat libero eu turpis tincidunt iaculis. In vel aliquam est, at porta neque. Donec hendrerit ultrices consectetur. Integer feugiat massa quis arcu ullamcorper, tristique facilisis massa feugiat. Phasellus risus mauris, pretium vitae tortor non, tristique fringilla tortor. Aliquam volutpat eros ac risus consectetur, quis gravida erat lobortis. Quisque id rutrum enim, sed fermentum nisl. Nulla volutpat turpis ac ligula venenatis maximus. In non dui ante. Sed ullamcorper quis nisi.