Biacore X100 reference portal
Assay setup for kinetics and affinity analysis
Concentration range
The range of analyte concentrations for optimal kinetic and affinity determination is related to the affinity constant for binding of analyte to ligand. Results from the pilot experiments should indicate a suitable concentration range.
As a general rule, cover a 100-fold or greater range of concentrations (up to 1000-fold if possible), where the lowest concentration should show measurable binding rates but will probably not reach steady state. At least one and preferably more of the highest concentrations should reach steady state during the sample injection. In relation to the affinity constant for the interaction, the range should ideally cover 0.1-100x the equilibrium dissociation constant. (In most cases, the affinity constant is not known, and the appearance of the sensorgrams from assay development must be used for guidance.)
If the analyte concentration is chosen correctly, it is possible to obtain an estimate of the kinetic constants from a single sample injection. However, the terms ka, C and Rmax in the evaluation model are intercorrelated: for example, an error in the analyte concentration C will result in a corresponding error in the calculated rate constant ka without affecting the quality of the fit. Determination from a series of analyte concentrations, and at multiple ligand levels if possible, is more robust and is generally recommended. Determination of affinity constants from steady-state response levels, on the other hand, always requires measurement over a series of analyte concentration.
Kinetic measurements
Calculation of kinetic constants requires significant curvature in both the association and dissociation phase of most or all of the sensorgrams. For screening purposes, adequate estimates of kinetic constants can be obtained from analysis of just two zero-subtracted curves (either two sample concentrations over one ligand spot or one sample over two spots with different ligand levels).
The analyte concentration series for robust kinetic determination should ideally comprise at least five concentrations with at least one of them in duplicate in addition to a zero concentration sample, over a range such that the highest concentration approached steady state during the sample injection while the lowest concentration still gives a measurable signal. Bear in mind that assays where the same sample solutions are injected over different ligands, either in the same or different flow cells, may need to use a wider range of analyte concentrations to cover suitable ranges for the different ligands.
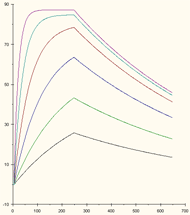 A suitable concentration range covers a wide range of observed binding rates.
|
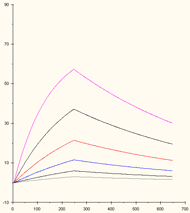 This concentration range is too low: none of the binding curves reaches steady state.
|
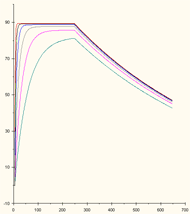 This concentration range is too high: the binding curves are clustered together.
|
Affinity measurements
For affinity measurements, the sensorgrams should reach a steady state during the association phase. If this is not the case, the calculated constants will not be correct, although they may give a rough indication of the actual values.
Affinity constants are determined from the relationship between the equilibrium binding level (Req) and the analyte concentration. At least 5 analyte concentrations are required for reliable interpretation of the data: two or more of the concentrations should be high enough so that the steady state binding levels Req approach saturation of the surface (Rmax). If the range of concentrations is too small, the curvature of the Req against C plot cannot be determined satisfactorily and the calculated KD value will be uncertain.
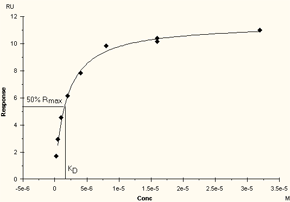 The highest concentration used should be at least twice the calculated KD-value for robust determination. On a plot of Req against C, the KD-value is equal to the concentration that gives 50% of the maximum response.
|
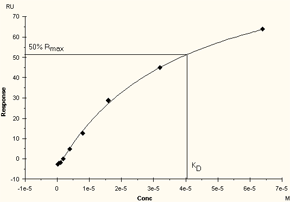 If the concentration range is too low, the plot of Req against C will not approach a maximum value and the determination of KD will be uncertain.
|
Purity of samples
Samples for kinetic and affinity analysis should preferably be as pure as possible, to ensure that the analysis is not complicated by sample heterogeneity or by contaminants that interfere in one way or another with the interaction being studied. It is possible to obtain kinetic or affinity estimates from unpurified samples (for example, characterizing monoclonal antibodies directly in hybridoma culture supernatant), but it is important to bear potential sources of interference in mind when interpreting the results.
Buffer for dilution
Careful matching of the refractive index between samples and running buffer contributes significantly to the robustness of both kinetic and affinity measurements. Bulk refractive index changes at the beginning and end of the sample injection can easily obscure rapid binding processes, and inaccuracies in reference subtraction can introduce unnecessary complications in the data fitting procedures, particularly if bulk shifts are large and sample binding responses are low. For preparing concentration series from stock sample solution, make sure that you use a dilution protocol that results in the same buffer conditions at all sample concentrations. In particular, avoid parallel dilution of bulk refractive index components (such as salt or stabilizing additives like glycerol) with the sample. If significant concentrations of bulk refractive index components cannot be avoided in the final samples, add the same concentration to the running buffer to match the refractive indices.
Dilution of samples
Careful preparation of the sample concentration series is important for the reliability of the kinetics, since all evaluation approaches depend on analyzing the response levels as a function of analyte concentration. Make sure that the pipettes used in preparing concentration series are correctly calibrated, and that sources of variation between samples in the series are eliminated as far as possible.
When preparing a concentration series, use multiples of a fixed volume so that you can use a single pipette setting for all dilutions. Samples that are prepared from different stock solutions or using different pipettes may have different systematic errors in concentration. If all samples are prepared by serial dilution using the same pipette, any errors of calibration in the pipette will be propagated evenly throughout the series. The resulting concentrations will be incorrect in absolute terms, but relative concentrations will be maintained: the curve fitting will not be affected, but the reported value for the affinity constant will be incorrect.
Make sure that all samples are carefully matched in buffer composition both within concentration series and with running buffer. The bulk refractive index effect should not exceed 400 RU. Include a zero-concentration sample in the analyses. This provides a complement to reference subtraction, and helps to confirm that buffers are correctly matched.
Kinetic analysis requires a reference surface to correct for bulk response. In multi-channel systems with serial flow cells, the reference surface should be placed upstream from the measurement surface.
Reference subtraction
Biacore instruments work by measuring refractive index changes close to the sensor surface. Any differences in refractive index between sample and running buffer will contribute to the response observed during sample injection. A bulk refractive index contribution is typically an immediate shift up or down in the response at the start of the injection, and a corresponding shift in the opposite direction at the end of the injection. Subtracting the response from a blank reference surface will help to eliminate this bulk refractive index contribution.
A schematic illustration of the principle of reference subtraction is shown below
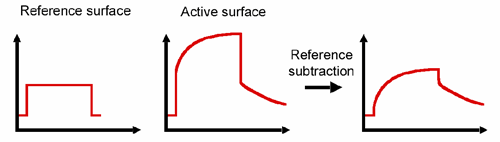
Principle of reference subtraction.
There may be other components in the reference response in addition to bulk refractive index contribution, for example non-specific binding of sample to the reference surface will generate a response. Non-specific binding may show time-dependent association and dissociation characteristics, in contrast to the immediate shift associated with a bulk refractive index contribution. Subtracting the reference response will not correct fully for non-specific binding if it is not identical on the reference and active surfaces.
Reference surface
The reference surface serves two functions in a Biacore assay:
- It allows reference subtraction of bulk response changes that arise from differences in refractive index between samples and buffer.
- It provides a control for non-specific binding of the sample with the sensor surface.
The choice of reference surface is to some extent determined by the design of the experiment, and three different approaches may be used in preparing the reference surface:
- A protein (or other appropriate molecule for non-protein ligands) that does not interact with analyte may be immobilized on the reference surface to a level similar to that of the ligand. For example, a mutant in the active site may be used. This provides the best reference since the properties of the surface mimic the ligand most closely.
- The reference surface may be activated and then deactivated (using for example EDC/NHS followed by ethanolamine for amine coupling), to reduce the negative charge density on the carboxymethyl dextran.
- An unmodified reference surface provides a control for interaction of the sample with the unmodified sensor surface but does not mimic the charge properties of the active surface.
Ligand level
The active surface for kinetic measurements is designed so that the maximum analyte binding capacity Rmax is low. This will facilitate measurement of fast on and off rates by reducing analyte transport limitations. For kinetic analysis, the amount of immobilized ligand should be kept low, so that the maximum response (Rmax) from analyte binding is typically in the region of 30 RU or lower.
Estimate the amount of ligand you need to immobilize from the results of the pilot experiments. The theoretical analyte binding capacity of the surface in RU is given by
Rmax = ligand level * (MWanalyte/ MWligand) * binding stoichiometry
In practice, the maximum binding capacity is usually lower than the theoretical value because the ligand preparation is not 100% active.
The ligand level is less critical for affinity measurements, since the steady-state response is not affected by mass transport limitations. Higher ligand levels are in fact preferable for affinity measurements since analyte response values will be higher and steady state response levels can be measured with more confidence.
For both kinetics and affinity, more robust determination is achieved if measurements on two different ligand levels are evaluated in combination. For kinetic measurements, ligand levels should be as low as possible, to keep mass transport limitations to a minimum. What this means in terms of response units of immobilized ligand will depend on the relative sizes of the ligand and analyte. Use this rule of thumb for the lower ligand level when you use two levels of the same ligand: the higher level should be at least 2 times higher.
Ligand capture or immobilization
Both kinetics and affinity determinations can be performed with the ligand immobilized or captured on the sensor surface. Even with robust and reproducible capture procedures, however, the ligand level may vary slightly between cycles if a capturing approach is used. This can be accommodated in kinetic evaluation by setting Rmax to a local parameter (so that a separate value for Rmax is estimated for each curve). However, evaluation of steady-state affinity assumes a constant Rmax for each measurement. Before using a capture approach for affinity determinations, make sure that the capture level is reproducible from cycle to cycle.
Panel 2 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 3 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.

Panel 4 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 4 header 2
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header 2
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header 3
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.