Biacore X100 reference portal
Assay design for antibody screening
Characterizing the antibodies produced
Injection of antigen after the antibodies are captured on the surface serves two purposes:
- A positive response confirms that the antibodies captured on the RAM-Ab have the correct specificity.
- The extent of antigen binding and the shape of the binding sensorgram give an indication of the kinetics and/or affinity of the antibody-antigen binding.
Assay verification
Conditions for capture of MAbs on anti-mouse immunoglobulin antibody and regeneration of the surface are well established: however, cell culture supernatants should be checked for possible interfering factors. The most common interference is seen as non-specific binding of sample matrix components to the surface. Inject antibody-free cell culture supernatant over the surface to detect non-specific binding.
Running buffer
Recommended running buffer for antibody screening assays is Hepes-buffered saline with 0.05% Surfactant P20 (HBS-EP+, available from Biacore). If you prepare your own running buffer, use physiological salt concentrations and include surfactant.
Sample preparation
Hybridoma culture supernatants, centrifuged or filtered to remove any particles, may be injected directly or diluted in running buffer, according to the expected concentration of antibodies in the supernatant. Pilot experiments may be necessary to establish suitable dilution levels.
Antigen over the captured MAbs should be in running buffer at a moderate to high concentration.
Report points for evaluation can be corrected in two ways:
- Adjustment for the molecular weight of the sample compound
- Correction for drift in the positive and/or negative control responses
The corrections are applied to report points. Report point corrections are to some extent interdependent (correction for drift is applied to the current report point values, and will change if the status of adjustment for molecular weight is changed).
Molecular weight adjustment
The response is directly proportional to the mass concentration change on the surface, so that adjustment for molecular weight is needed if responses from different analytes are to be compared on a molar basis. The adjustment is performed by dividing the measured response by the molecular weight. The result is then scaled up by a factor of 100, to keep the values within the same orders of magnitude as unadjusted response values. (This scaling simply presents the adjusted responses at levels to which the user is accustomed, and is in no way essential to the principle of molecular weight adjustment.)
Notes: The molecular weight adjustment corrects the response for different molecular sizes of analytes, but does not take account of other properties of the analytes that may affect the specific response.
Molecular weight adjustment should not be used for surface competition assays, since the measured response in this assay format derives from the competing analyte and not from the lead compound.
The example below illustrates the effect of molecular weight adjustment on the binding levels in a hit selection assay. The substances tested ranged in molecular weight from 201 to 765 Da. Clearly, molecular weight adjustment can have a decisive effect on assessment of relative binding levels.
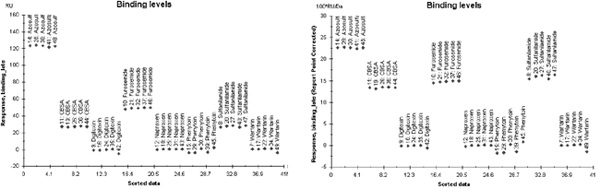
Illustration of the effect of molecular weight adjustment. These two panels show the binding levels for a set of control compounds, before (left) and after (right) molecular weight adjustment. The plots are sorted by sample name.
Adjustment for controls
Adjustment of report points for controls allows compensation for drift in the positive and/or negative control response throughout the assay. The commonest situation where this adjustment should be used is probably gradual loss of activity from the surface, resulting in a downward drift in the positive control values.
The adjustment for controls normalizes all report points to a function defined by two control samples, where the lower control value is set to zero and the higher value to 100. The normalization values are calculated for each cycle from a curve fitted to the control sample responses plotted against cycle number. Normally, you will select one positive and one negative control.
Screening assays are evaluated by comparison of the response levels obtained from the sample injection between different samples.
Classification of binding strength
With the same concentration of different analytes, the response value gives a direct indication of the binding strength. The simplest approach to evaluating screening is to compare response levels from one report point.
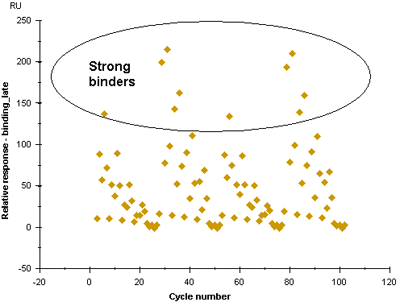
A plot of cycle number against report point values on the y-axis.
Classification of kinetic properties
Comparison of response levels measured at different times during sample injection and dissociation can also give an approximate indication of kinetic parameters for the binding that complements the classification of samples in terms of binding strength.
A plot of report point binding_late against stability_late can provide a rough visualization of kinetic properties for all samples in a single plot. The binding_late report point reflects the level of binding reached during the association phase, while stability_late reflects the extent of dissociation during a fixed period. Non-binders among the samples cluster close to the origin. Points with a significant binding_late response but low stability_late represent samples that bind but dissociate rapidly, while points with high stability_late values bind more strongly.
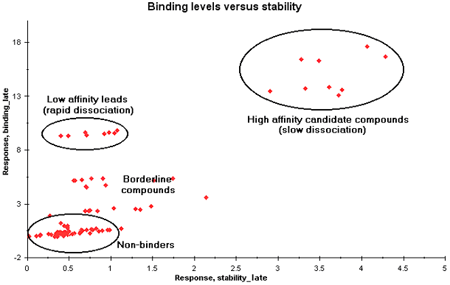
In a plot of binding_late against stability_late, high values on the y-axis indicate rapid association while high values on the x-axis indicate high stability (slow dissociation).
Screening provides two main types of data:
- “Yes/no” answers for binding of individual compounds
- Ranking of a set of compounds in terms of relative binding levels
An additional goal of the screening is to obtain preliminary kinetic or affinity information on the antibody clones, since these parameters can be important deciding factors in selection of antibodies for both biopharmaceutics and biotechnology applications.
The essential difference from screening for small molecules apart from analyte size is that the antibody concentration is not known.
Assay format
Screening for antibody production from hybridoma cultures can in principle be set up in a format analogous to small molecule screens, with antigen immobilized on the surface and cell culture supernatant injected as sample. A more convenient and informative approach is however to immobilize anti-mouse immunoglobulin antibody on the surface as a capturing molecule, inject the cell culture supernatant over this surface to capture the MAbs, and then inject antigen in solution to confirm the MAb identity and to provide information on antibody-antigen characteristics. Although this is a somewhat more complicated approach and the sample throughput is lower compared with immobilized antigen, there are a number of advantages:
- Immobilization and regeneration of anti-mouse immunoglobulin antibody is well characterized, so the assay development work involved in finding immobilization and regeneration conditions for antigen is eliminated.
- Anti-mouse immunoglobulin antibody interacts with the constant region of MAbs, so that the response obtained from injection of the supernatant over anti-mouse immunoglobulin antibody gives a direct indication of the concentration of MAb produced by the hybridoma clone, independently of the antibody-antigen binding characteristics.
- The antigen response can be normalized to the level of captured MAb, so that an indication of the kinetic and/or affinity properties of the MAb can be obtained directly from the first screening. This can help to eliminate cell clones early in the development procedure that produce MAbs unsuitable for the intended application.
- Suitable antibodies for use as capturing molecules are available from Biacore.
Panel 2 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 3 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.

Panel 4 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 4 header 2
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header 2
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.
Panel 5 header 3
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Repellendus aliquid tempore natus voluptates repudiandae, eos dolorem libero inventore, quod incidunt, asperiores. Reiciendis itaque enim pariatur, blanditiis minima autem quo a nulla, obcaecati quis, excepturi atque ab rerum! Sequi molestias vel eum, hic, perspiciatis eius suscipit reprehenderit molestiae vitae similique.